FAQs About Participation in IATS
What is IATS?
The I nfant A phakia T reatment S tudy ( IATS ) is a randomized, controlled, multi-center clinical trial sponsored by the National Eye Institute (NEI) of the National Institutes of Health (NIH) . This clinical trial will try to determine whether infants with a unilateral, congenital cataract are more likely to develop better vision after cataract extraction surgery if they are treated with a contact lens or if they receive an intraocular lens (IOL) implant.
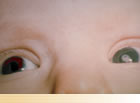
What is a unilateral congenital cataract?
Unilateral means ‘on one side'. Congenital is any condition which is present or develops soon after birth. A cataract is an opacity (cloudiness) in the lens of the eye.
Thus, a unilateral congenital cataract is a cataract that is present in just one eye at birth.
What is cataract extraction surgery?
Cataract extraction surgery is the surgical removal of the clouded lens (cataract) from the eye.
What is intraocular lens implantation?
This surgical procedure places a tiny prescription lens, known as an intraocular lens (IOL), inside the eye to replace the eye's natural lens which is clouded (cataract).
Which patients can participate in IATS?
The infants in this study must have a visually, significant congenital cataract, equal to or greater than 3mm in only one eye. The child must be 28 days to less than 7 months of age, and least 41 post-conception weeks at the time of the cataract surgery. The infant must not suffer from glaucoma, uveitis, retinal and optic nerve disease, nor have any other ocular disease in the other eye. Measurements of the eye at the time of the cataract extraction surgery will be the final step in determining the eligibility of the infant as a participant in the study. The parents or legal guardians of the child are required to sign an informed consent form and must also agree to be contacted by staff of the Data Coordinating Center (DCC) in Atlanta , GA.
Where can I find an IATS Clinical Center ?
IATS is being conducted at 12 clinical centers and research institutions throughout the USA . Each institution has a Principal Investigator (PI) and a Coordinator. The Eye Center of Emory University is the Clinical Coordinating Center (CCC) for IATS. The PI and National Coordinator who head the whole study are located there. The DCC includes Emory faculty and staff from the departments of Biostatistics and Epidemiology of the Rollins School of Public Health as well as the department of Psychiatry of the School of Medicine . You can get more information by calling the Clinical Center closest to you.
Can I choose the treatment my child will get?
No. The treatment will be randomly assigned. Random means each treatment is assigned by chance, similar to the flip of a coin. While you may feel a little uneasy about not choosing your child's treatment, rest assured that this study would not have been permitted if the doctors, the sponsoring body, the NEI , and the Food and Drug Administration (FDA) did not believe that both treatments are safe and work well.
How long will my child have to stay in the study if they are enrolled?
Each child enrolled in this study will be required to see the study doctor (PI) at least once every three months for the duration of the funding of IATS which is about 4 years.
What benefits do my child and I receive for enrolling in IATS?
The study offers a strong support system to help parents during the challenges of following a treatment plan for any child who has had a cataract removed. This plan may include the wearing of a contact lens and/or glasses and patching.
Participation in the study involves careful medical monitoring by the study teams who are experts in this specialized field. The safety of your child will be closely monitored by an IATS physician monitor, the NEI and its board of experts the Data and Safety Monitoring Committee (DSMC), and the FDA . You will receive a Caregiver Resource Notebook as a reference guide to keep you informed and educated on your child's condition and this particular study. Finally, looking to the future, you and your child will help doctors and researchers to determine the best treatment for other infants born with a cataract in one eye.
Are there any risks to my child for being in the study?
The known risks for this condition are present whether a child is enrolled in this study or not. There are risks associated with having cataract surgery at any age such as glaucoma and retinal detachment. The surgery of babies under anesthesia is also associated with risks. These risks include drug reactions and, very rarely, brain damage or death. Bacterial keratitis and lens loss are some problems associated with the wearing of contact lens. More post-operative complications are reported with the use of IOLs compared to the use of contact lens.
Is there an additional financial burden associated with enrolling in the study?
No. The cost of cataract surgery removal and follow-up office visits are not covered by the study and must be paid for by your insurance and you. However, the study will provide the IOL, contact lenses, glasses and patches at no charge. You will be given $100 every 3 months to reimburse you for travel costs associated with the post-surgery visits and study activities. An additional $100 will be given if all visits and activities are completed at the end of each study year.
What is the NEI?
The National Eye Institute ( NEI ) is one of the 27 institutes of the NIH. The NEI conducts and supports research that helps prevent and treat eye diseases and other vision disorders. The NEI provides the funding for IATS and also oversees the research, safety, and conduct of the study.
What is the NIH
The National Institutes of Health ( NIH ) is the steward of medical and behavioral research in the United States . It funds and oversees scientific studies at universities and research institutions across the nation. The NIH is comprised of 27 institutes and centers.
What is the FDA?
The Food and Drug Administration ( FDA ) is the federal body that is charged with protecting the public health by assuring the safety and security of drugs, medical devices, food, cosmetics, and products that produce radiation. It also helps to speed innovations that make medicines and foods more effective, safer, and more affordable.
Where can I get more information on IATS?
You can contact the PI and Coordinator at the Clinical Center closest to you. You can also contact the staff at Emory University Clinical Coordinating Center if you prefer.