About IATS Clinical Trial
Treatment Options
Purpose of the IATS Clinical Trial
Monitoring of the IATS Trial
Participation in the IATS Trial
What Treatment will My Child Receive?
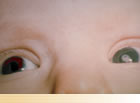
What treatment is best for your child? Since the cataract (clouded lens) is not letting enough light into the eye, the lens has to be surgically removed. Removing the lens lets light into the eye, but then something else has to do the lens's job of focusing the image. One way to do this is to fit the infant's eye with a contact lens. The strength of the contact lens can be changed as the baby's eye grows. Those people caring for the baby will learn how to insert, remove, and clean the contact lens. A patch is applied over the stronger eye at certain times during waking hours. If a patch is not placed over the stronger eye, the eye with the contact lens starts to let the other eye do all the visual work. The vision in this "lazy eye" will be poor, a condition known as amblyopia.
A second way to replace the lens is for the surgeon to place a plastic lens inside the eye (intraocular lens) at the time the cataract is removed. By having the plastic lens inside the eye the baby will always have his / her vision corrected. The strength of this lens cannot be changed as the child grows since it is on the inside of the eye. Glasses will be given to the child as the eye grows and needs different corrections. The stronger eye is patched at certain times during waking hours to prevent amblyopia from developing in the eye with the intraocular lens.
The Infant Aphakia (without a lens) Treatment Study (IATS) is a research study designed to find out if one or the other of these treatments is better when the treatment is begun in a young baby less than 7 months of age who is born with a cataract in one eye. The National Eye Institute has paid for the study to be conducted in 12 U.S. institutions.
This clinical study will be closely monitored by the NEI, the Food and Drug Administration (FDA), and a specially formed board of scientists and eye experts. The surgeons and study team members are experts in this specialized field and are among the most experienced doctors in the country in treating babies who are born with cataracts.
Every study has specific guidelines (criteria) which must be met in order for someone to participate. For instance one of this study's entry requirements is that the infant must be less than seven months when the surgery is performed. If your doctor finds that your child has met the first set of IATS criteria then he / she may talk to you about this study. A written detailed description of the study and the requirements for participation (informed consent) will be given to you. You will be able to ask any questions that you have about the study. If you decide that you want your child to be in this study, you will sign the consent form. You have the right to stop your participation in the study at any time.
It is important to realize that if you choose to participate, you will not know which of the two treatments your child will receive. The treatment will be randomly assigned. Randomly means by chance, like a flip of a coin. Assigning your child's treatment in this way is the best scientific way for doctors to find out if one of the two treatments is better. At the time the cataract is being removed in surgery, the surgeon will examine your child's eye to make sure the remaining study criteria are met. If the criteria are met, your child will be in the study. At this time, the surgeon or a member of the surgical team will open a sealed envelope. A card inside the envelope will say whether the surgeon is to place an intraocular lens (plastic lens) inside the eye along with the cataract removal or to perform the cataract removal and fit your child with a contact lens about a week later. While it may feel uncomfortable not to be able to choose the treatment your child will receive, this study would not be done if the doctors did not believe both treatments are safe and work well. IATS is being done to find out if one of these treatments is better when begun in infants 7 months or younger.