LESSON 7
![]()
VALIDITY
7-1 Validity
Primary objective of most epidemiologic research - obtain valid
estimate of an effect measure of interest.
In this Lesson
·
Three general types of validity problems
·
Distinguish validity from precision
·
Introduce term bias
·
How to adjust for bias.
Examples of Validity
Problems
Potential problems with studies
· Imperfections in
study design
· Imperfections in
data collection
· Imperfections in
analysis
No imperfections = Valid
Imperfections = Bias
Bias results in distortion of results
Validity versus Precision
Validity & precision - influenced by 2 different errors
·
Systematic error affects validity
· Random error affects precision.
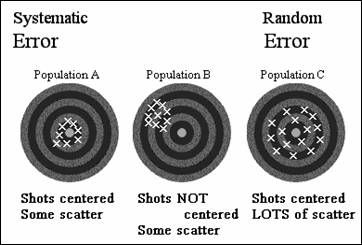
Valid = No systematic error (i.e., unbiased)
The bull’s eye usually not known, therefore difficult to determine
extent of bias.
Precision: a lot of spread = poor
precision “imprecise”
little spread = good
precision “precise”
Precision reflects sampling variability.
A Hierarchy of Populations
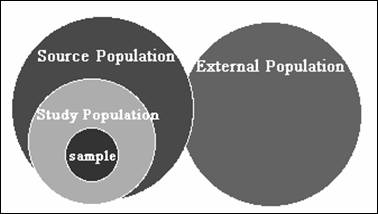
v The sample - collection
of individuals from which study data have been obtained.
v
The study population - the individuals that our sample actually
represents (typically those we can feasibly study).
v
The source population - group of interest about which the
investigator wishes to assess an exposure-disease relationship.
v The external population
- group to which the study has not been restricted but to which the
investigator wishes to generalize.
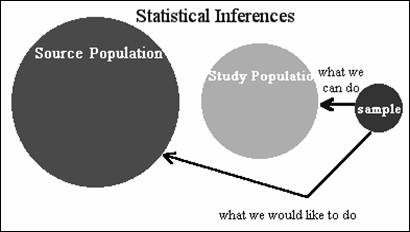
We would often like to generalize our conclusions to a different external
population.
Internal versus External
Validity
Target shooting illustrates difference between internal and external
validity.
· Internal validity considers
whether or not we are aiming at the center of the target. If shooting off target, then study is not
internally valid.
|
|
|
Internal validity - drawing conclusions about source population based on study
population.
External validity – conceptually concerns a different target. We might imagine this external target being
screened from our vision.

External validity - applying conclusions to an external population beyond the study's
restricted interest; subjective, less quantifiable than internal validity.
7-2 Validity
(continued)
Quantitative Definition of
Bias
A bias can be defined quantitatively in terms of the target
parameter of interest and measure of effect actually being estimated in the
study population.
A study that is not internally valid is said
to have bias.
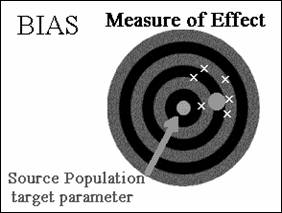
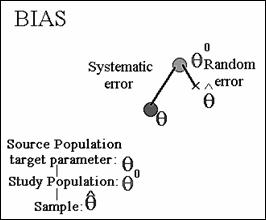
Target parameter - Greek letter θ (“theta”). We want to estimate the value
of θ in the source population.
θ0 the measure of effect in the study population.
![]() (“theta-hat”) denotes
the estimate of our measure of effect obtained from the sample actually
analyzed.
(“theta-hat”) denotes
the estimate of our measure of effect obtained from the sample actually
analyzed.
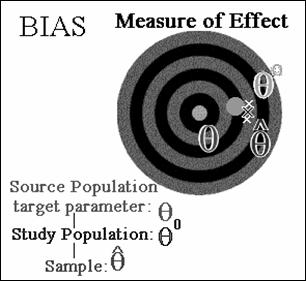
Differences between ![]() and θ0
the result of random error.
and θ0
the result of random error.
A difference between θ0 and θ is
due to systematic error.
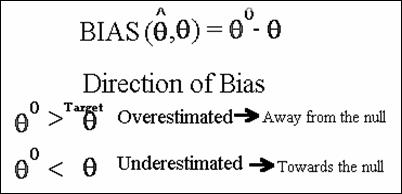
v Bias (![]() ,θ) = θ0 - θ
,θ) = θ0 - θ
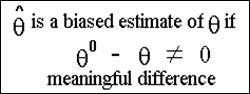
The not equal sign should be interpreted as a meaningful
difference from zero.
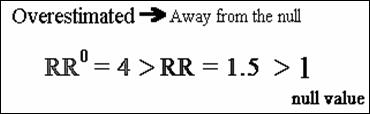
Direction of the Bias
The precise magnitude of bias can never really be quantified,
however, the direction of bias can often be determined.
· The target parameter can be overestimated
(bias away from the null)
· The target parameter can be underestimated
(bias towards the null).
Examples



Switchover bias

Positive vs. negative bias – do not worry about these terms
What Can be Done About Bias?
Three general approaches for addressing bias:

1. Design stage -
minimize or avoid bias. Avoid selection bias by including/excluding
eligible subjects, by
·
Choice of source population
·
Choice of the comparison group
2. Analysis stage -
determine presence or direction of possible bias
Also, account for confounding in analysis.
3. Publication stage -
Potential biases typically described in "Discussion" section. For selection
and information bias, is subjective, judgment expected given inherent
difficulty in quantifying biases.

